Why Does Silicon Dioxide Have High Melting Point
Why does silicon dioxide have a high melting point. Melting and boiling points.
Silicon has a very high melting point due to its giant covalent structure.

. A Silicon dioxide has a very high melting point. This is due to the strength of Si-O-Si binds in the lattice. 1 The melting point of the elements going across the Periodic Table from sodium to argon A increases steadily.
Silicon has a very high melting point due to its giant covalent structure. Thus the melting temperature of silicon is higher as it exists as a giant covalent structure so strong covalent bonds have to be broken in order to melt it whereas phosphorus exists as a simple covalent structure so weaker London forces have to broken in order to melt it. Glass is made from silicon dioxide.
Despite being covalent why does silicon dioxide have a high melting point. Glass melts at a lower temperature than silicon dioxide. Why is melting point of the oxides high and why do magnesium and aluminium oxide have a higher melting point than sodium oxide.
Silicon dioxide is suitable for lining furnaces because of itsvery high melting point of 1600 to 1725 degrees celcius. Also Know why does silicon have a higher melting point than phosphorus. In addition carbon is able to form very strong covalent bonds with other atoms which also contributes to its high melting and boiling points.
A lot of energy is needed to break the strong covalent bonds throughout the structure. Why does silicon dioxide have such a high melting point. Since covalent bonds require more energy to overcome than van der waals SiO2 requires a higher temperature to melt.
Melting and boiling points. The giant structures the metal oxides and silicon dioxide will have high melting and boiling points because a lot of energy is needed to break the strong bonds ionic or covalent operating in three dimensions. The carbon atoms are held together by strong covalent bonds which.
Explain why silicon dioxide has a high melting point above 1600ºC whereas carbon dioxide is a gas at room temp and pressure. Why does silicon dioxide have such a high melting point. A lot of energy is needed to break the strong covalent bonds throughout the structure.
Why do diamond and graphite both have high melting points. The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds. SiO2 has a high melting point.
A lot of energy is needed to break the strong covalent bonds throughout the structure. Covalent bonds stronger in SiO2 than in SiCl4 so more energy isneeded to break bonds. Since Diamonds contain more covalent bond than Silicon Dioxide and these bonds requires heatenergy to break Diamond has a higher melting point than Silicon dioxide.
The oxides of phosphorus sulphur and chlorine consist of individual molecules - some small and simple. Why does carbon has a high melting point and high boiling point. Silicon has a very high melting point due to its giant covalent structure.
Both diamond and graphite have a giant molecular structure. Melting and boiling points. The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds.
Because the silicon and oxygen atoms are held by strong intermolecular forces. Silicon dioxide has a giant molecular structure with strong covalent bonds resulting in a high melting point. Simply so why does silicon have a higher melting point than sodium.
Why does silicon dioxide have a high melting point. These oxides tend to be gases liquids or low melting point solids. Carbon has a high melting point and high boiling point because it is a strong non-metallic element.
Other substances are added to silicon dioxide to make glass. It is sohigh because of. Carbon dioxide contains individual molecules of carbon dioxide held together by weak intermolecular forces.
The giant structures the metal oxides and silicon dioxide will have high melting and boiling points because a lot of energy is needed to break the strong bonds ionic or covalent operating in three dimensions. Why does silicon dioxide have a high melting point. SiO2 has higher melting point.
The large structures the metal oxides and silicon dioxide have high melting and boiling points because a large amount of energy is needed to break the strong bonds ionic or covalent operating in three dimensions. Why does silicon dioxide have such a high melting point.

Ch 4 The Structure Of Matter Section 1 Compounds Molecules Ppt Download

Quick Answer Why Does Silicon Carbide Have A High Melting Point Seniorcare2share

Based On Their Structures Explain Why Sodium Oxide Silicon Dioxide And Carbon Dioxide Have Different Melting Points Here Are 6 Real Student Answers Ppt Download

22 2 The Oxides Of Elements In Period 3 Flashcards Quizlet

Why Does Silicon Have A High Melting Point Seniorcare2share

Why Does Silicon Have A High Melting Point Seniorcare2share

Carbon Dioxide Is A Gas But Silica Is A Solid Because Youtube
Why S The Melting Point Of Silicon More Than Sulfur Despite The Fact That Sulfur Consists Of 8 Atoms In A Molecule Where Silicon Has Only 4 Quora

Based On Their Structures Explain Why Sodium Oxide Silicon Dioxide And Carbon Dioxide Have Different Melting Points Here Are 6 Real Student Answers Ppt Download

Why Do Atoms Bond They Want To Have A Full Outer Electron Shell This Is Why Oxygen That We Breathe In Is O 2 Chlorine Gas Is Cl 2 Etc Metalnon Metal
![]()
Giant Covalent Compound Properties Ppt Video Online Download

Intermolecular Forces Generalizing Properties Ppt Video Online Download
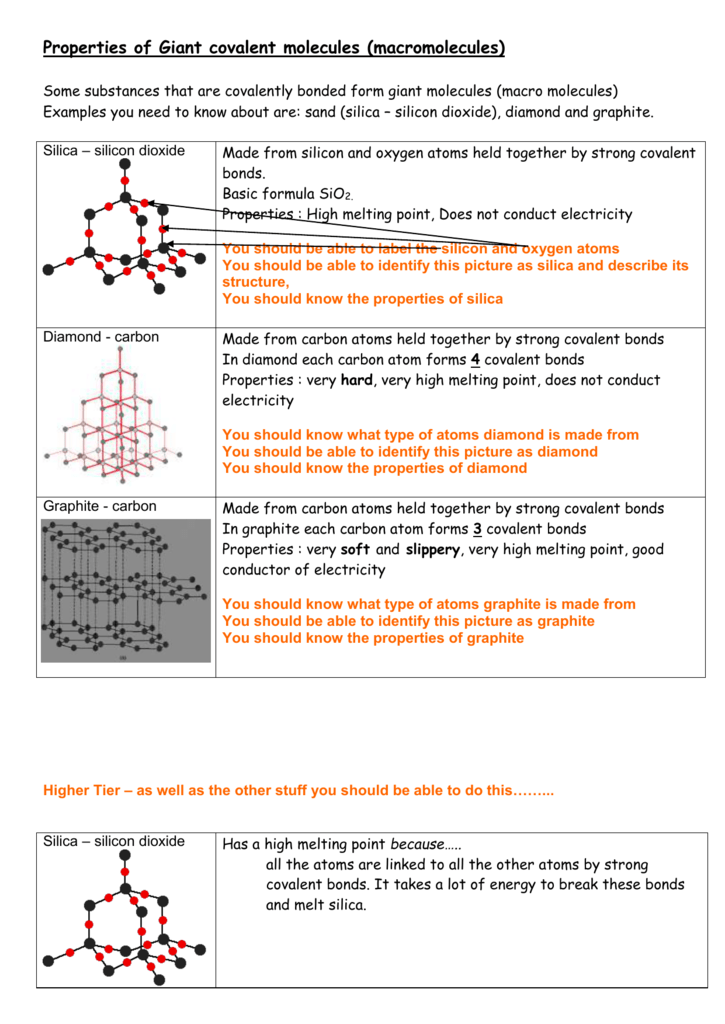
Properties Of Giant Covalent Molecules Macromolecules

Why Does Silicon Have A High Melting Point Seniorcare2share
![]()
Macromolecules Covalent Network Solids Last Part Of Topic Ppt Download

Why Is Carbon Dioxide A Gas While Silicon Dioxide A Solid Quora
At Room Temperature Why Is Sio2 Solid And Co2 Gas Quora
Why Does Silicon Dioxide Have A Higher Melting Point Than Silicon Tetrachloride In Terms Of Structure And Bonding Quora
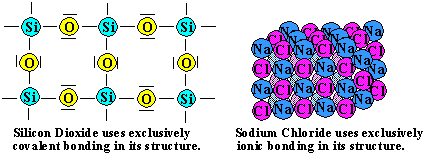

Comments
Post a Comment